Pelage Pharmaceuticals Redefines Hair Loss Treatment with Regenerative Therapy
FIFTIERS | Life Begins at 50. La vida comienza a…
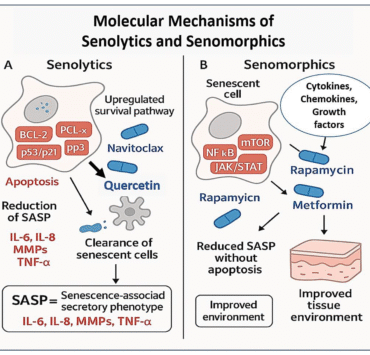
Biotech firm Pelage Pharmaceuticals has closed a $120 million Series B funding round to advance PP405, a breakthrough regenerative therapy designed to reverse androgenetic alopecia by reactivating the body’s own stem cells.
Unlike traditional hair loss treatments that merely slow shedding, PP405 targets the biological root cause—dormant hair follicle stem cells—stimulating them to restart natural growth. In early Phase 2a trials, 31% of patients achieved more than a 20% increase in hair density after just eight weeks, with no significant adverse effects reported.
Developed from research at the University of California, Los Angeles (UCLA), the therapy represents a new frontier in regenerative dermatology and the rising dermato-longevity field, which focuses on maintaining youthful skin and hair as markers of cellular vitality.
“The future of dermatology lies in regeneration, not coverage. PP405 brings us closer to truly reversing hair loss at its biological core,” said Dr. Katherine Lee, Chief Scientific Officer at Pelage.
With the fresh capital, Pelage plans to move into Phase 3 trials in 2026 and prepare market entry across the U.S., Europe, and Asia. The treatment is also expected to drive growth in the premium longevity-aesthetic segment, increasingly popular among consumers over 45.
For FIFTIERS readers, this breakthrough symbolizes the merging of science, aesthetics, and well-being: biotechnology at the service of confidence, vitality, and a longer, healthier life.
Discover more from FIFTIERS
Subscribe to get the latest posts sent to your email.