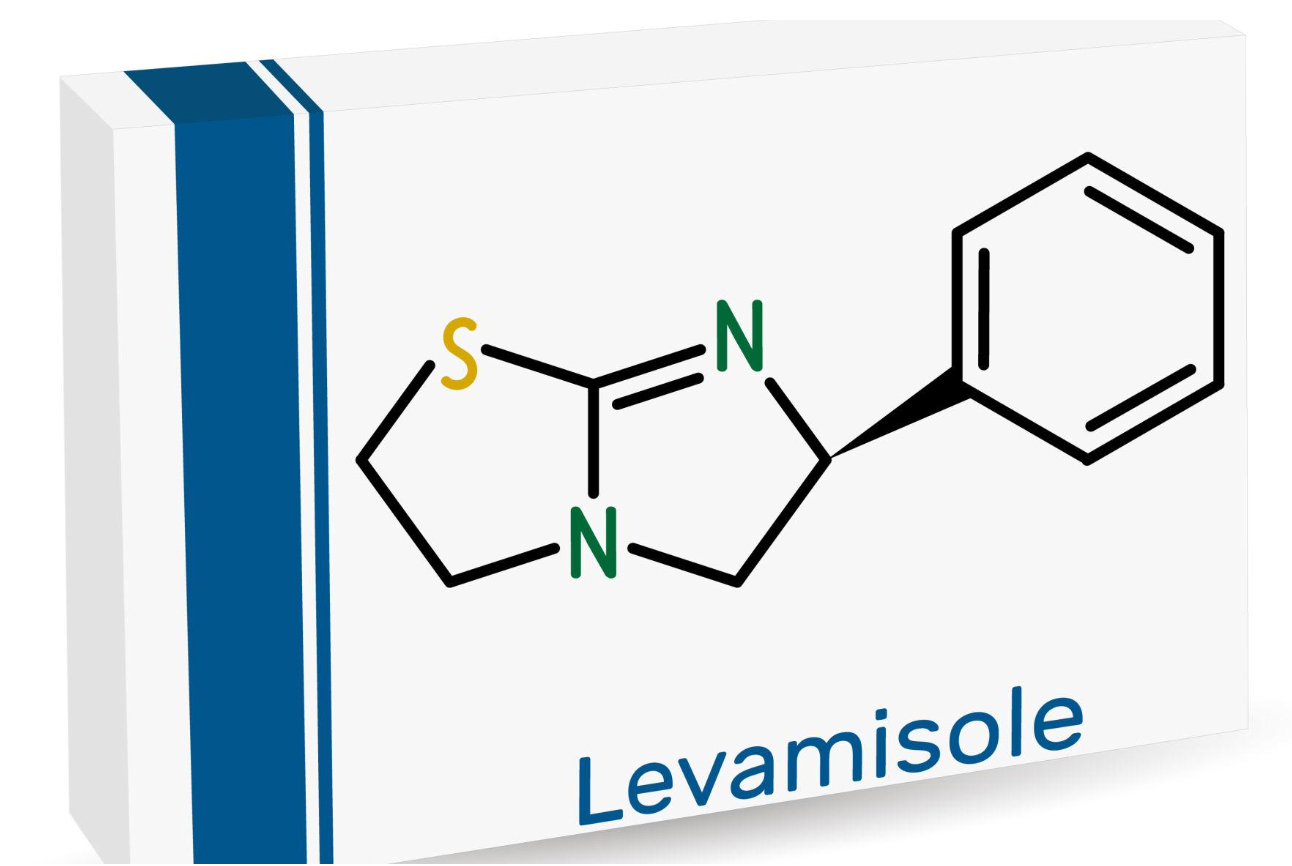
EMA Recommends Withdrawal of Levamisole Medicines Over Serious Neurological Risk
FIFTIERS | Life Begins at 50. La vida comienza a…
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency has recommended the withdrawal of marketing authorisations for medicines containing levamisole across the European Union after concluding that their benefit–risk balance is no longer favourable. The decision follows a review of reported cases of leukoencephalopathy, a rare but potentially serious condition affecting the brain’s white matter.
What Was Levamisole Used For?
Levamisole was originally developed as an anthelmintic agent, meaning it was used to treat intestinal parasitic infections, particularly those caused by nematodes (roundworms). Over time, researchers identified its immunomodulatory properties, which led to additional medical applications.
Its main uses historically included:
-
Treatment of certain intestinal parasitic infections.
-
Use as an adjuvant therapy in oncology, particularly in combination with chemotherapy for colorectal cancer in previous decades.
-
Management of selected immune-related or dermatological conditions in specific clinical contexts.
In recent years, however, its use in Europe had become limited, and in most indications safer and more effective therapeutic alternatives were already available.
Why the Review Was Initiated
According to PRAC’s assessment, cases of serious neurological adverse events were reported, including leukoencephalopathy occurring even after short-term exposure. Symptoms may include cognitive impairment, motor disturbances, confusion, and progressive neurological decline.
Given the severity of these potential effects and the availability of alternative treatments, the committee determined that the risks outweigh the benefits.
Implications for Patients and Healthcare Professionals
The PRAC recommendation will now be reviewed by the Committee for Medicinal Products for Human Use (CHMP) before a final decision is adopted by the European Commission. In the meantime, healthcare professionals are advised to reassess patients currently receiving levamisole and consider appropriate alternatives.
Patients are strongly encouraged not to discontinue treatment on their own, but to consult their healthcare provider for personalised medical advice.
A Broader Perspective in an Ageing Society
As populations live longer and polypharmacy becomes more common—particularly among individuals over 50—continuous drug safety monitoring remains essential. This case illustrates how European regulatory systems are designed to reassess medicines throughout their lifecycle and act decisively when new safety evidence emerges.
The final regulatory decision will follow formal validation procedures, but the message is already clear: patient safety remains the overriding priority when scientific evidence identifies serious risks.
Discover more from FIFTIERS
Subscribe to get the latest posts sent to your email.